Cindy Tung / Dr. Susan Gauthier - Week 3
I had the opportunity to be in the neurology ICU during their morning rounds where physicians and nurses brief the attending on each patient on the floor and discuss treatment plans moving forward. After that, they went to each of the patient's rooms to check on their progress and briefly speak with the patients. While some patients were progressing towards improvement and even getting discharged, some had worsening conditions beyond treatable.
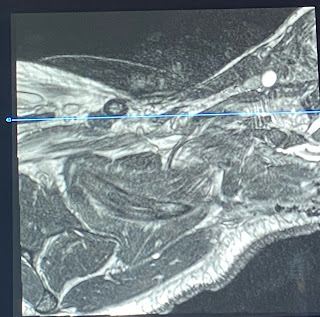
In the neurology reading room, I shadowed radiologist Elcin Zan as she looked through patient images including PET and MRI. During MR-guided PET reconstruction, the reconstruction happens inside the scanner whereas MR-guided is a reformation of an existing image. A key difference I noticed between neurology reading versus cardiac reading is that much of the analysis of the brain comes from the expertise of the physician. Other than measuring the size of lesions or parts of the brain, any irregularities are pointed out manually by the radiologist. On the other hand, cardiology analysis stems from computing stroke volume and blood flow following the time progression of one cardiac cycle. This difference most likely stems from the motion of the heart beating during scans compared to a still image of the brain in a head coil during a scan. The following image shows cortical injury from an invasive surgery as a result of demyelination. The lesions are identified by a bright/hyperintensity lump in FLAIR.
I also shadowed Laura while she performed tumor cell culturing in the lab. Cells are grown in a flask containing red fluorescent media (growth source) and stored in a freezer (~ -20°C) to grow. After a couple days, the flask is washed with PBS, and cells are detached using Trypsin. To count the number of cells, trypan blue is used to stain the cells, then 10 μL are examined under this machine on a microscope slide.
After cell count, red media is added back to the tube and centrifuged down to form pellets of cells. The cells are then resuspended in PBS and kept in an ice bath.
The mice injection procedure is as follows:
1. Use isoflurane to sedate them
2. Measure their weight
3. Shave the lower abdomen area
4. Inject 10 μL (contains 1 million cells) into the mice
5. tag the ears for identification


Comments
Post a Comment